Water hardness is caused almost entirely be calcium and magnesium ions.
Other di- and tri-valent metals have a similar effect, but are usually not present in high enough concentration in potable waters to cause problems.
Hardness increases soap consumption in laundries and causes scale in boilers.
| Method | Range (mg/L) | Steps (mg/L) | Approx. Number of Tests |
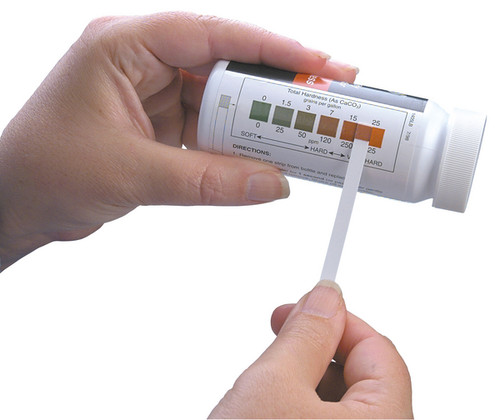
| Test Strip | 0-425 | 0, 25, 50, 120, 250, 425 | 50 |
| (0-25gpg) | (0,1.5, 3, 7, 15, 25 gpg) | ||
| 1 gpg = 17.1 - mg/L or 17.1 ppm | |||
gpg - grains per gallon